 |
Plant Physiology (Biology 327) - Dr. Stephen G. Saupe; College of St. Benedict/ St. John's University; Biology Department; Collegeville, MN 56321; (320) 363 - 2782; (320) 363 - 3202, fax; ssaupe@csbsju.edu |
 |
Plant Physiology (Biology 327) - Dr. Stephen G. Saupe; College of St. Benedict/ St. John's University; Biology Department; Collegeville, MN 56321; (320) 363 - 2782; (320) 363 - 3202, fax; ssaupe@csbsju.edu |
Diffusion, Osmosis
& Water Potential Quiz
| a. | The plant cell will become: | a. larger b. smaller c. not change |
| b. | The weight of the plant cell will: | a. increase b. decrease c. not change |
| c. | The concentration of the sucrose solution in the beaker will: | a. increase b. decrease c. not change |
| d. | The turgidity of the plant cell will: | a. increase b. decrease c. not change |
| e. | The osmotic potential of the sucrose solution will become: | a. more negative b. less negative |
| f. | There will be a net movement of water from the: | a. cell to the solution b. solution to the cell |
| g. | After a few hours the cell is removed. A drop of sucrose (-4.0 MPa) placed in the solution will: | a. float b. sink c. hover & disperse |
| h. | The refractive index of the sucrose solution will: | a. increase b. decrease c. not change |
| i. | The cell will likely plasmolyze: | a. false b. true |
| j. | The initial concentration of the sucrose solution (in molality) is: | |
| k. | Assume that at equilibrium, the water potential of the cell becomes -3.5 MPa. Thus, the concentration of the sucrose solution (in molality) in the beaker at equilibrium is: |
Osmometer Question: The diagram represents a simple osmometer. The membrane is freely permeable to water by not solute (sucrose). The membrane is inelastic. Inside the membrane is a solution of 0.1 molar sucrose.
|
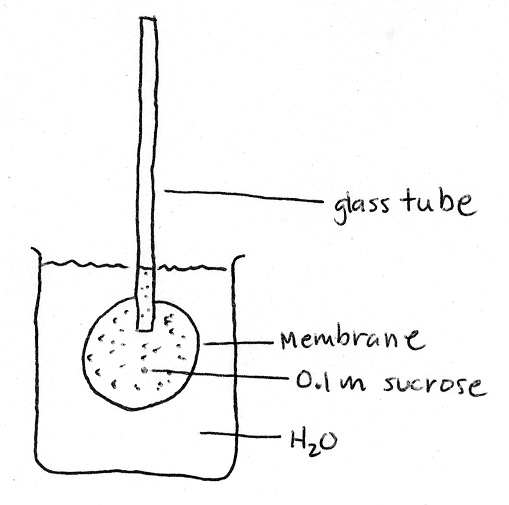 |
Some
Multiple Choice Questions:
1. A cell whose internal osmotic concentration is 0.3 osmoles/liter is placed in a solution that is 0.5 osmoles/liter. The solution is:
a.
Isoosmotic
to the cell
d. Isotonic to the cell
b.
Hypoosmotic to the cell
e. Hypertonic to the cell
c.
Hyperosmotic to the
cell f.
Hypotonic
to the cell
2. A cell is placed in a
solution and swells. The solution
is:
a. Isoosmotic
to the cell
d. Isotonic to the cell
b. Hypoosmotic to the cell
e. Hypertonic to the cell
c. Hyperosmotic to the
cell f. Hypotonic
to the cell
3.
A cell is placed in a solution and the cell swells.
The solution is probably, but not necessarily:
a. isoosmotic to the cell
b.
hypoosmotic to the cell
c.
hyperosmotic to the cell.
Short Answer Questions: Using water potential terminology, explain why:
Calculation Question:
Determine the water potential of a cell if Ψp = 0.3 MPa and
Ψs = -0.5
MPa. Calculate the pressure in a cell if Ψw = -0.1 and Ψs = -0.2? Calculate
the osmotic potential of a cell (Ψs) if Ψw = -0.1
and Ψp = 0.8.
Measuring Water Potential - A Simulation
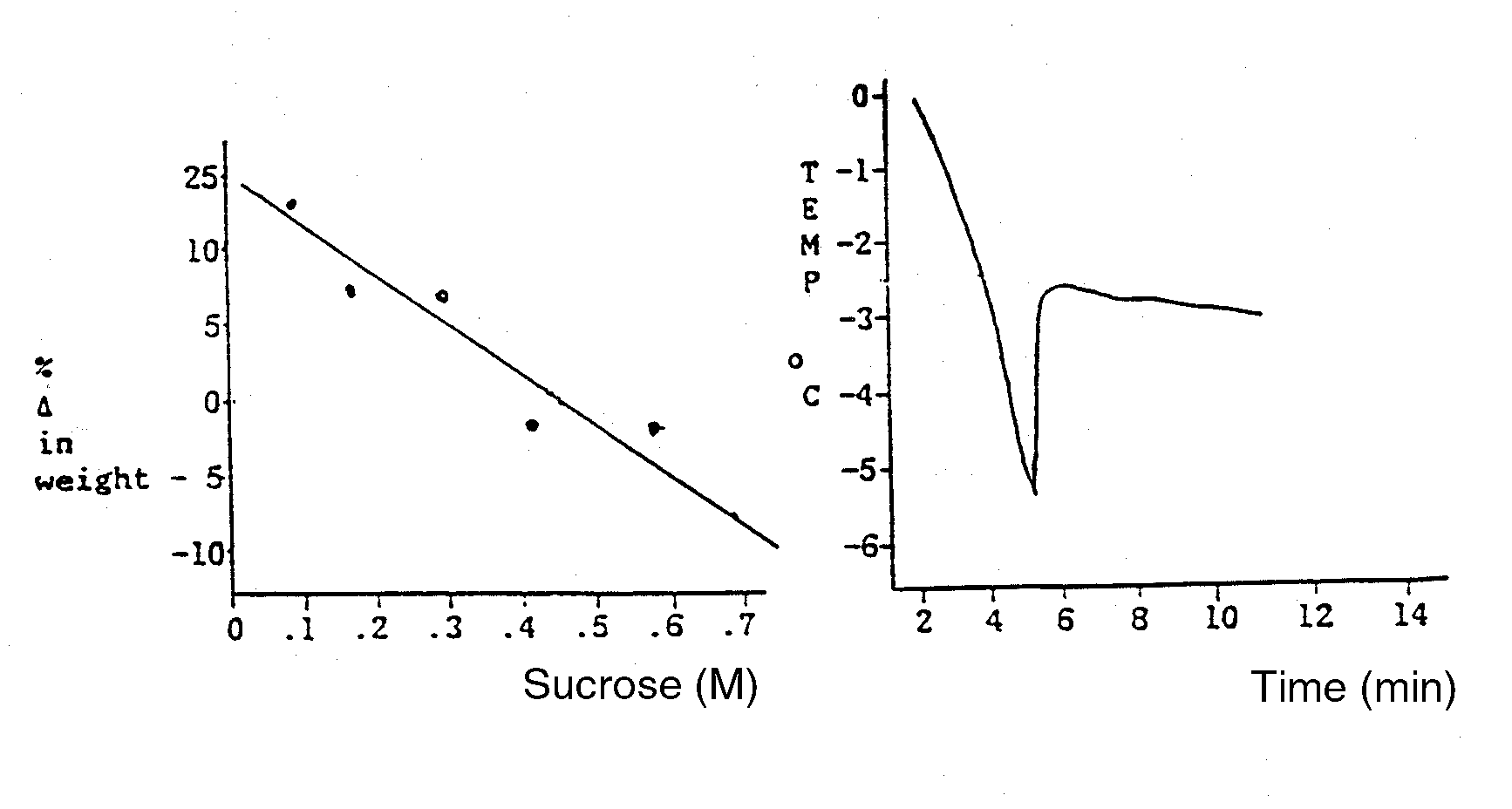
A plant physiologist wanted to study the water relations of a turnip. She prepared
cores from the turnip with a core borer and then determined their change in weight after
incubation in a graded series of sucrose solutions at 25 C. These data are presented in Fig
1. She also ran a freezing point depression experiment on the turnip sap and
these data are
also plotted in Fig 2. Using these data, answer the following questions:
- What is the water potential (MPa) of the turnip cells (show your work)?
- What is the osmotic potential (MPa) of the turnip cells (show your work)?
- What is the pressure potential (MPa) of the turnip cells (show your work)?
 |
||
| Fig 1. Gravimetric Data | Fig 2. Freezing Point Data | |
For an enlarged version of these diagrams, Click here (tiff file)
Hofler Diagram
Question:
Which of the
following is the appropriate label for the "X" axis?
a. Water, pressure
or solute potential (Ψ, MPa)
b. Relative cell volume (Δ
V/V)
Which is the appropriate label for the Y axis?
a. Water, pressure
or solute potential (Ψ, MPa)
b. Relative cell volume (Δ
V/V)
Write the labels on the graph.
Which line best reflects the change in water potential that would occur as a cell volume changes? Label this line Ψw.
Which line best reflects the change in pressure potential that would occur as a cell volume changes? Label this line Ψp.
Which line best reflects the change in solute potential that would occur as a cell volume changes? Label this line Ψs.
What do you conclude from this graph?
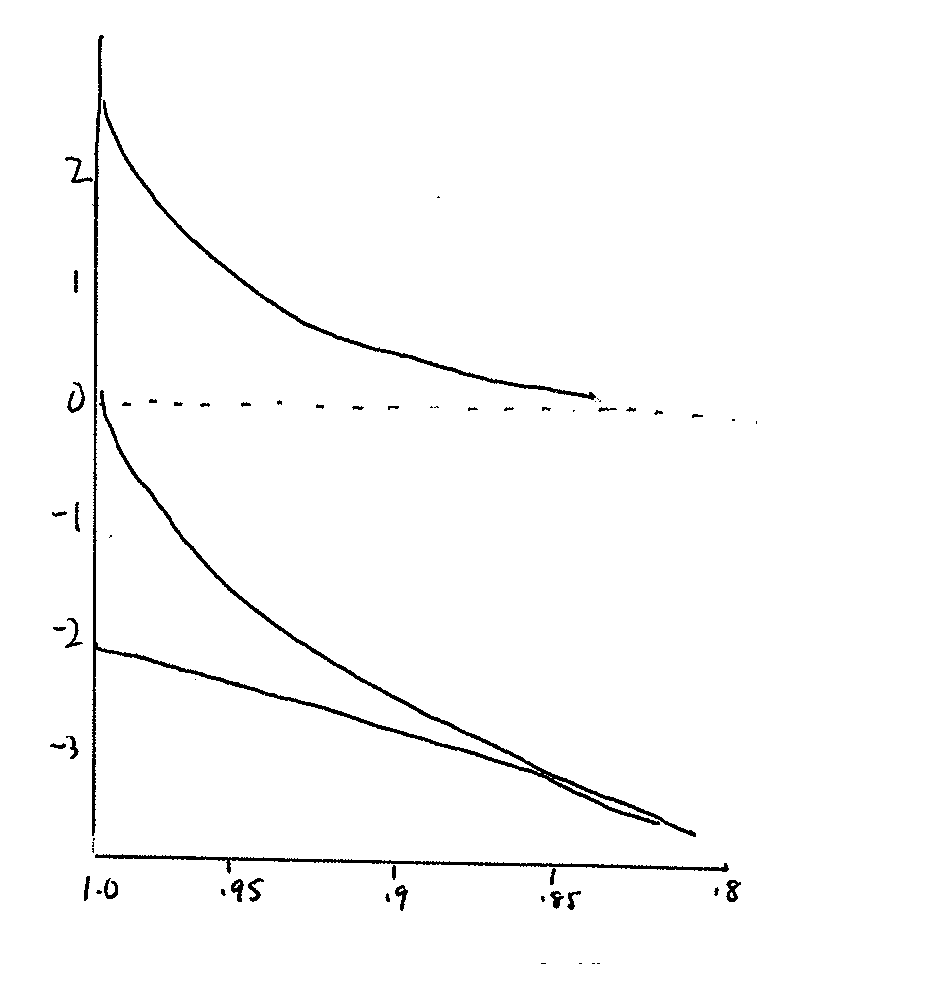
Diffusion Coefficient Question: To calculate the time it takes for a substance to diffuse a particular distance, use the equation time (s) = L2/Ds where L is the diffusion length (in meters) and Ds is the diffusion coefficient. For glucose, Ds = 10-9m2s-1.
Calculate the time it will take a glucose molecule to diffuse through a
single cell wall if the diameter of the wall is 100 �m.
Calculate the time it would take a glucose molecule to diffuse 1.0 meter.
| | Top | SGS Home | CSB/SJU Home | Biology Dept | Biol 327 Home | Disclaimer | |
Last updated:
02/24/2009 � Copyright by SG
Saupe