 |
Plant Physiology (Biology 327) - Dr. Stephen G. Saupe; College of St. Benedict/ St. John's University; Biology Department; Collegeville, MN 56321; (320) 363 - 2782; (320) 363 - 3202, fax; ssaupe@csbsju.edu |
 |
Plant Physiology (Biology 327) - Dr. Stephen G. Saupe; College of St. Benedict/ St. John's University; Biology Department; Collegeville, MN 56321; (320) 363 - 2782; (320) 363 - 3202, fax; ssaupe@csbsju.edu |
Determining Protoplast Concentration with a Hemocytometer
Background Information:
In order to calculate the anthocyanin concentration, we will need to
know the number of protoplasts per mL of our protoplast preparation. The number of cells
(or other particles) in a sample is routinely determined with a hemocytometer.
This
device, which was originally developed to count blood cells, is essentially a modified
microscope slide that has two chambers. Each chamber is ruled into nine, 1 mm
squares and these, in turn, are further divided. A typical ruling is shown
below:
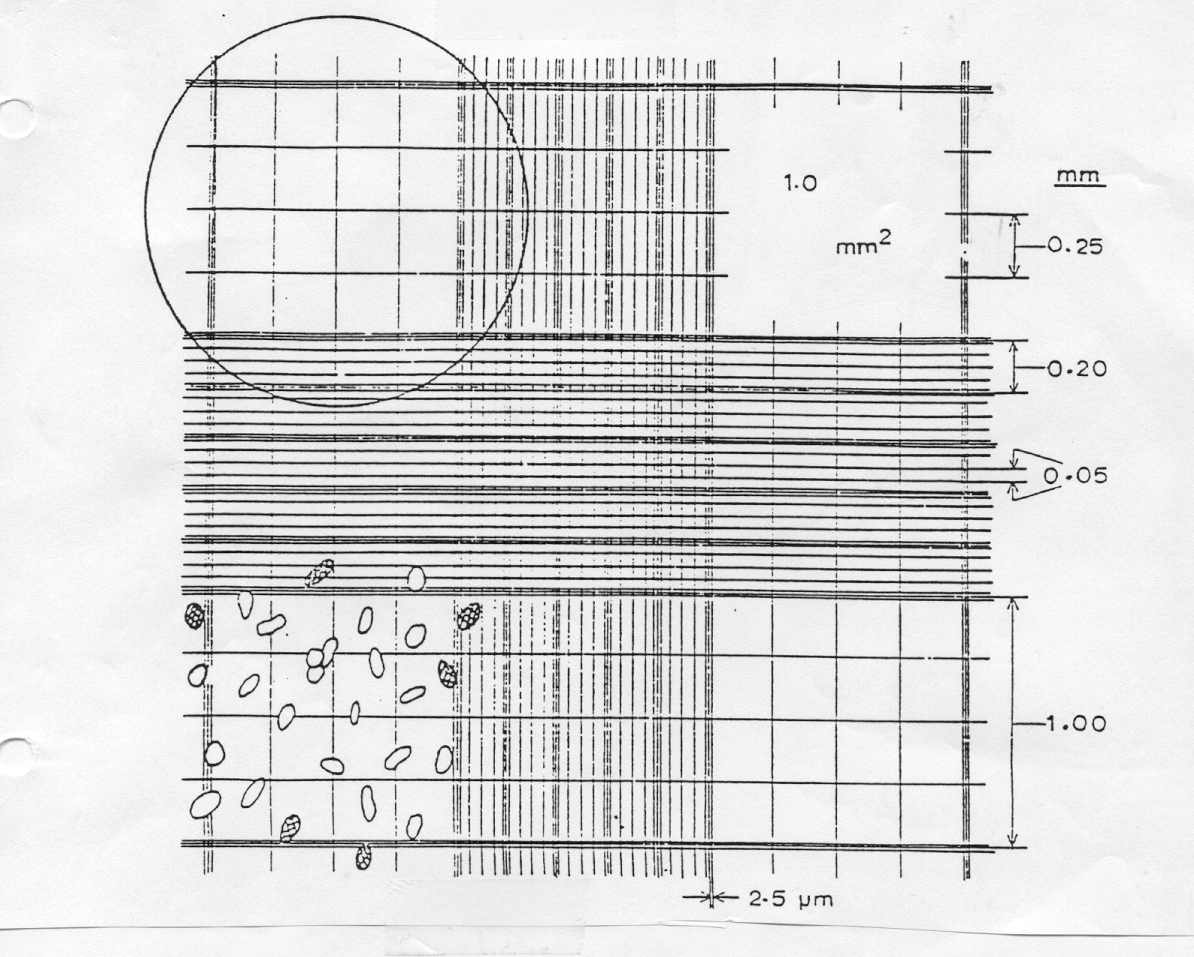
A cover glass placed over the chambers is supported 0.1 mm above the ruled area. The volume over any sized square can easily be determined once the size of the square to be counted is determined. Thus, when the chamber is filled, the volume of the sample over each of the 9 larger squares is:
0.1 mm3 (1 mm x 1 mm x 0.1 mm) or 1 x 10–4 ml
(remember that 1 mL = 1 cm3 = 1000 mm3)
The number of particles per unit volume in a sample can therefore be determined simply by counting the number of particles in several, appropriate-sized squares.
Question(s): How many protoplasts mL-1 are in the protoplast preparation?
Hypothesis: Depending upon yield, we can expect large numbers of protoplasts, at least 106 per mL.
Protocol:
Data:
cells/ml = cells/square x 1 square/volume (mm3) x 1000 mm3/ ml x dilution factor
| Table 1: Hemocytometer Data | |
| grid size counted | 0.1 mm x _____ mm x _____mm |
| volume of grid counted (mm3) | |
| dilution (if any) | |
| Table 2: Protoplast density in a partially purified suspension of Red cabbage protoplasts measured with a hemocytometer | ||
Grid |
protoplasts grid-1 | protoplasts ml-1 |
1 |
||
2 |
||
3 |
||
4 |
||
5 |
||
6 |
||
7 |
||
8 |
||
9 |
||
10 |
||
11 |
||
12 |
||
13 |
||
14 |
||
15 |
||
16 |
||
| mean +/- std | ||
Analysis & Conclusions:
| | Top | SGS Home | CSB/SJU Home | Biology Dept | Biol 327 Home | Disclaimer | |
Last updated:
01/07/2009 � Copyright by SG
Saupe